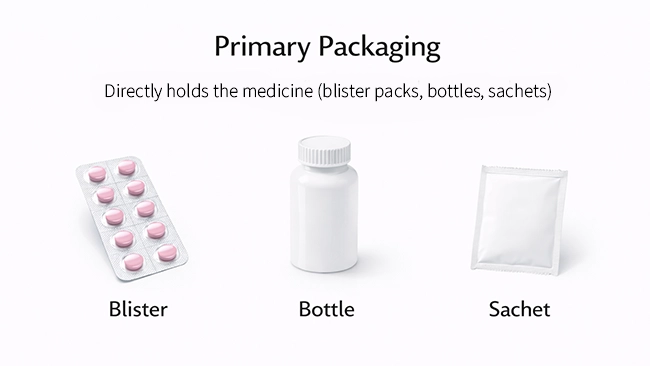
Primary packaging is the first layer that directly contains a medicine (or directly seals in the dose). It sounds like a packaging detail, but it often determines whether a product stays stable, safe, and consistent from production to patient use.
For many tablets and capsules, a “small” change—switching from blister to bottle, changing a foil, using a different liner—can change moisture exposure, oxygen contact, or physical protection. That’s why many manufacturers treat primary packing as more than “a container”: it’s a protection system that can affect shelf life, breakage risk, and the confidence behind batch release decisions.
A simple way to think about it: if you removed everything except the layer that actually contains and protects the dose, what’s left is usually the primary packing. Getting that definition right makes it much easier to compare formats, materials, and (later in this article) the typical packaging equipment and high-level line flow that go with each choice.

It is the packaging that directly contains the medicine and forms the immediate barrier between the product and the outside world. In most cases, it’s also the layer that creates the “seal” that protects the dose.
Common examples include:
● Blister packs: the formed blister holding each tablet/capsule plus the lidding material (often foil) that seals it.
● Bottles: the bottle plus the closure system (cap, liner, induction seal where applicable). In other words, the “bottle system,” not just the bottle shape.
● Sachets / pouches: the film structure that directly holds and seals the powder, granules, or single dose.
● Vials / ampoules: the container itself, and for vials the stopper + seal as part of the closure system.
● Pre-filled syringes: the barrel and closure components that keep the drug sealed and protected.
What usually doesn’t count as primary packing:
● Cartons (paper boxes), leaflets, bundles/shrink wrap, and most outer labeling layers. These are typically secondary or tertiary packaging—still important, but not the first protective barrier.
If primary packing is the “dose protector,” secondary packaging is often the “dose organizer and communicator.”
● Primary packaging focuses on protection: barrier to moisture/oxygen/light, seal integrity, compatibility, and physical protection.
● Secondary packaging focuses on presentation and information: labeling space, tamper evidence features, patient instructions, and unit organization (like cartons holding multiple blisters or bottles).
A practical example:
● A blister pack is primary packaging because each blister is a sealed barrier around the dose.
● The carton holding that blister is usually secondary packaging because it doesn’t directly seal the dose, but it carries labeling, leaflets, and physical support during distribution.
This distinction matters because when packaging changes, it often triggers more attention around stability risk and documentation expectations. Regulators treat the container-closure concept as part of the product’s quality story, not just a marketing choice. (U.S. Food and Drug Administration)
Many solid-dose products (including common supplements) are moisture-sensitive. Moisture can change tablet hardness, accelerate degradation, or cause capsules to soften or stick. Primary formats like high-barrier blisters or well-designed bottle systems help slow that moisture exposure.
Oxygen is a slow, invisible enemy for certain actives and excipients. A package that is “good enough” for one product may not be good enough for another with higher oxidation sensitivity. The key idea is not perfection—it’s reducing exposure enough to hit the intended shelf life.
Light sensitivity is easy to underestimate because the product can look fine while the active slowly degrades. Amber bottles, opaque blisters, and cartons can all play roles here, but the true “first barrier” is still the layer touching the dose.
Even when chemistry is stable, physical damage can create quality problems: broken tablets, powdering, chipped edges, or capsules losing a clean finish. Primary packing also helps maintain dose integrity through shipping and handling—especially for coated tablets, fragile shapes, and multi-SKU products.
Here’s a simple comparison you can use when you’re thinking in “format first” terms (not material chemistry). This is also where “what it protects” starts to connect naturally to downstream packaging equipment.
|
Primary format |
Common medicines |
What it protects best |
Typical trade-offs |
Equipment implications |
|
Blister packs (unit-dose) |
Tablets, capsules |
Great moisture/oxygen control (with the right lidding); dose-by-dose protection; strong tamper evidence |
Design and material choices matter; format changes require tooling; graphics space is limited on the blister side |
Often pairs with a blister packaging machine, then cartoning for secondary pack |
|
Bottles + closure system |
Tablets, capsules, gummies |
Convenient for consumers; flexible count sizes; more labeling space |
Moisture/oxygen control depends heavily on closure/liner and headspace; tablet abrasion can be a concern |
Often pairs with a counting & bottling line (counting/filling + capping), then optional cartoning |
|
Sachets / pouches (single-dose) |
Powders, granules, some effervescents |
Good single-dose protection; portability; portion control |
Seal quality and film choices matter; less convenient for multi-dose routines |
Often pairs with stick packing machine / sachet packing machine; optional cartoning depending on presentation |
|
Vials / ampoules |
Liquids, injectables |
Strong container protection |
Format is less flexible; often higher packaging/handling complexity |
Typically associated with sterile fill-finish systems (beyond this article’s scope) |
|
Pre-filled syringes |
Biologics, injectables |
Dosing convenience; reduced handling |
High component quality expectations |
Specialized sterile systems; not comparable to solid-dose lines |
If your products are mostly tablets/capsules, blisters and bottles usually carry the bulk of practical decisions
Materials are where people often get overwhelmed, so keep the goal simple: materials determine barrier behavior and compatibility risk.
● Plastics (used in bottles, some blister films, and pouches) vary widely in moisture barrier and oxygen barrier. “Plastic” isn’t one thing; it’s a family.
● Foils and multi-layer films (especially in blisters and sachets) can provide much stronger barriers than many plastics, but they come with trade-offs in cost, recyclability, and forming/sealing windows.
● Glass (common in vials and some bottles) is often viewed as a strong barrier material, but the overall protection still depends on the full container closure system, not the container alone.
A useful beginner mindset: format decides the job, materials decide how well the job is done. That’s why primary format and material are often discussed together in stability planning, especially for products intended for longer shelf life or tougher distribution conditions.
Regulators and experienced manufacturers often talk about a container closure system because real protection comes from the whole system, not one component. For example, a bottle without a suitable liner or seal strategy can behave very differently from the same bottle with a better closure design.
Key concepts:
● Container closure system (CCS): the container + all components that create and maintain the seal (cap, liner, foil seal, stopper, etc.).
● Seal integrity: does the seal remain reliable over time and across expected shipping/handling conditions?
● Compatibility: can the product and packaging components interact in ways that affect quality (for example, adsorption, migration, or odor transfer)?
● Change sensitivity: packaging changes can trigger re-evaluation because small component differences can shift performance or risk assumptions.
Most oral solids begin life as either tablets or filled capsules. That upstream choice usually points to equipment like a tablet press machine or capsule filling machine earlier in the process, but this article focuses on what happens when the product is ready to be packed.
Once the dose is ready, the packaging decision tends to lock in a typical packaging flow:
● Blister-first (unit-dose) flow: doses are arranged and sealed into blister cavities, then moved into secondary packaging such as cartons. In equipment terms, you’ll usually see a blister packaging machine by a cartoning machine (and sometimes printing/inspection steps depending on product and market requirements).
● Bottle-first flow: doses are counted (or otherwise portioned) into bottles, then closed and labeled, with optional cartoning for retail presentation or added protection. In equipment terms, this commonly involves a tablet counting machine (or a broader counting & bottling line) and then downstream operations like capping and optional cartoning.
This connection helps you avoid two common mistakes:
1. deciding on a primary format without considering whether the line can maintain consistent output and protection, and
2. assuming a packaging change is “just a packaging change” when it may alter stability risk and documentation expectations.
Mistake 1: “A stronger barrier is always better.”
Sometimes yes, but not always. Higher barrier can raise cost, limit format flexibility, or create trade-offs in sustainability goals. The “best” barrier is the one that reliably supports shelf life and use conditions.
Mistake 2: “Primary packaging is separate from stability.”
In practice, stability planning often treats packaging as part of the product’s protection model. Container closure and storage conditions are directly related to the shelf life.
Mistake 3: “Switching blister ↔ bottle is a simple marketing decision.”
It can be a branding decision, but it’s also a protection system change—different moisture exposure patterns, different physical handling, and different evidence expectations.
Mistake 4: “If it seals today, it will seal for the full shelf life.”
Seal reliability is about performance over time and distribution stresses, not only a single moment in production.
A few trends keep showing up across both pharma and supplements:
● Sustainability pressure: more interest in recyclable structures and material reduction, which can create new barrier trade-offs.
● More information on-pack: traceability, clearer instructions, and market-specific labeling needs can shift how secondary packaging is used, even when primary stays the same.
● Higher expectations for consistency: as SKUs grow, manufacturers care more about predictable packaging performance that scales.
These trends don’t pick a single “winner” format, but they do push buyers to ask better questions about what the packaging must achieve.
If you’re new to packaging decisions, these questions are a solid starting point:
1. How sensitive is the product to moisture, oxygen, or light?
2. How fragile is the dose during shipping and everyday handling?
3. How will the customer use it—single-dose portability or multi-dose convenience?
4. What does your target market require for labeling, tamper evidence, or child resistance?
5. Which packaging flow fits your scale-up plan: blister-first or bottle-first?
If you can answer these questions clearly, you’ll usually avoid the most expensive packaging mistakes.
Primary packaging is the first protective layer surrounding the medicine—and for many products, it behaves like part of the product itself. Once you understand what counts as primary packing, you can compare formats and materials with a clearer goal: protect stability, maintain dose integrity, and support safe use.
Just as importantly, primary format choices naturally connect to a typical packaging flow and the machines that run it. Blister-first and bottle-first approaches each solve different problems well, and the “right” choice is the one that fits your product risks, use case, and scale-up needs.
1) How does primary packaging affect shelf life?
It controls exposure to moisture, oxygen, and light, and protects the dose physically. Different formats/materials can change the product’s stability risk profile.
2) What is a container closure system in simple terms?
It’s the container plus all parts that create and maintain the seal (cap, liner, foil seal, stopper, etc.).
3) What does seal integrity mean and why does it matter?
Seal integrity is whether the seal stays reliable during storage and distribution. A weak seal can undermine the barrier your product depends on.
4) How do you choose between blister and bottle for tablets or capsules?
Start with moisture sensitivity and dose protection needs, then consider user convenience and labeling requirements. Blisters often win on unit-dose protection; bottles often win on convenience.
l FDA — Container Closure Systems for Packaging Human Drugs and Biologics (guidance).