Dans l'industrie pharmaceutique, un secteur hautement réglementé, la presse à comprimés est un élément essentiel de la fabrication des formes posologiques solides. Ces équipements sophistiqués, communément appelés presses à comprimés, remplissent la fonction cruciale de transformer les formulations en poudre ou en granulés en comprimés de forme précise, présentant une taille, un poids, une dureté et des caractéristiques de dissolution constants. Le processus de sélection de ces machines à comprimés est devenu de plus en plus complexe en raison des progrès technologiques, des exigences réglementaires et de la diversification des formulations. Des presses à simple poinçon aux presses multicouches sophistiquées capables de gérer des systèmes d'administration de médicaments complexes, le marché offre de nombreuses options. Ce guide complet constitue votre feuille de route stratégique pour la sélection, fournissant des informations détaillées pour mener à bien le processus d'évaluation et prendre une décision éclairée, en adéquation avec vos besoins actuels et vos projets de croissance futurs.
Une presse à comprimés fonctionne selon le principe de l'application d'une pression mécanique contrôlée sur des poudres ou des granulés pharmaceutiques dans un espace confiné afin de créer des comprimés aux spécifications prédéterminées. Son mécanisme fondamental repose sur plusieurs composants intégrés fonctionnant en parfaite coordination : la trémie régule l'alimentation en matière, la cavité de la matrice définit les dimensions du comprimé et les poinçons appliquent la force de compression grâce au mouvement de rotation de la tourelle. Ce fonctionnement synchronisé se déroule en trois phases distinctes – remplissage, compression et éjection – qui se répètent rapidement pour atteindre les objectifs de production.
L'évolution de la technologie de compression a donné naissance à diverses configurations de machines, chacune conçue pour des applications spécifiques. Les presses à poinçon unique représentent la conception la plus simple, adaptée aux opérations à petite échelle, tandis que presse à comprimés rotative Les presses à comprimés multicouches dominent la production commerciale grâce à leur fonctionnement continu et leur capacité de production élevée. Pour les formulations plus complexes, elles permettent de combiner des principes actifs pharmaceutiques (API) incompatibles ou des profils de libération modifiée, tandis que les presses multicouches étendent encore davantage cette possibilité. De plus, des machines spécialisées existent pour des applications spécifiques telles que les comprimés effervescents ou les comprimés à croquer. Comprendre ces différences fondamentales est essentiel pour faire le choix le plus approprié, en adéquation avec les exigences de votre produit et vos objectifs de production.
Les caractéristiques de votre formulation de comprimés doivent guider le choix de votre machine. Pour les comprimés monocouches classiques contenant un seul principe actif, une presse à comprimés rotative simple offre généralement la solution la plus économique, alliant simplicité d'utilisation et fiabilité. Cependant, la complexité croissante des systèmes d'administration de médicaments exige des équipements plus performants. Les comprimés bicouches et multicouches nécessitent des presses spécialisées dotées de systèmes d'alimentation multiples et de mécanismes de compression distincts afin de maintenir la séparation du principe actif et d'assurer l'intégrité des couches. Ces machines sont indispensables pour les thérapies combinées ou les produits nécessitant une libération séquentielle du médicament.
Pour les comprimés de formes spécialisées, comme les comprimés effervescents qui nécessitent une force de compression élevée, ou les comprimés enrobés qui requièrent des caractéristiques de surface spécifiques, les spécifications de la machine doivent être soigneusement adaptées aux exigences du procédé. La catégorie croissante des comprimés orodispersibles (ODT) soulève des considérations supplémentaires, car ces formulations exigent souvent une porosité contrôlée et une dureté minimale. La connaissance des caractéristiques physico-chimiques de votre produit, actuelles et futures, garantit que la machine sélectionnée possède les capacités techniques appropriées.
Une planification précise de la production est essentielle pour déterminer la capacité machine appropriée. Pour les productions en petites séries, notamment les activités de R&D, la fabrication pour les essais cliniques ou les produits destinés à des marchés de niche, les presses à comprimés manuelles ou semi-automatiques offrent un rendement suffisant et une grande flexibilité pour le passage d'un produit à l'autre. Ces systèmes produisent généralement de plusieurs milliers à plusieurs dizaines de milliers de comprimés par heure, tout en permettant aux opérateurs de surveiller et d'ajuster précisément les paramètres de production.
Tableau : Guide de planification des capacités de production
| Scénario de production | Type de machine recommandé | Plage de sortie typique | Besoins en personnel |
|---|---|---|---|
| Recherche et développement / Développement de formulations | Perforateur simple ou mini-rotatif | 1 000 à 10 000 comprimés par heure | 1 opérateur |
| Fabrication pour essais cliniques | petite presse rotative pour la fabrication de comprimés | 10 000 à 50 000 comprimés par heure | 1 à 2 opérateurs |
| Produits de niche/spécialisés | Presse rotative moyenne | 50 000 à 100 000 comprimés par heure | 2 opérateurs |
| Commercial à grand volume | Machine de compression de comprimés rotative à grande vitesse | 100 000 à 1 000 000+ comprimés/heure | 2 à 3 opérateurs + superviseur |
À l'inverse, la production industrielle à grande échelle exige des presses à comprimés rotatives à grande vitesse, capables de produire de plusieurs centaines de milliers à plus d'un million de comprimés par heure. Ces machines comportent plusieurs stations de compression disposées sur une tourelle rotative, permettant un fonctionnement continu à haute vitesse. Lors de l'évaluation des besoins en capacité, il convient de prendre en compte non seulement les volumes de production actuels, mais aussi la croissance prévue, la planification du cycle de vie du produit et les perspectives d'expansion du marché. Le concept de TRS (Taux de Rendement Synthétique) devient alors crucial, car des facteurs autres que la simple vitesse – tels que le temps de changement de format, les besoins de maintenance et les pertes de rendement – ont un impact significatif sur la production réelle.

La fabrication pharmaceutique est soumise à un cadre réglementaire strict que le choix des machines doit prendre en compte de manière exhaustive. Les exigences des Bonnes Pratiques de Fabrication (BPF) actuelles imposent des caractéristiques de conception spécifiques, notamment l'utilisation d'acier inoxydable 316 pour les surfaces en contact avec le produit, des finitions polies facilitant le nettoyage et des conceptions éliminant les zones mortes où des matières pourraient s'accumuler. Ces caractéristiques préviennent la contamination et garantissent une qualité de produit constante.
Au-delà des caractéristiques physiques, les exigences réglementaires mettent de plus en plus l'accent sur l'intégrité des données et la validation des procédés. Les équipements doivent répondre à ces exigences grâce à des systèmes de surveillance intégrés qui enregistrent les paramètres critiques du procédé, les enregistrements électroniques étant conservés conformément à des réglementations telles que la norme FDA 21 CFR Part 11. Des fonctionnalités comme les systèmes automatisés de contrôle du poids, la surveillance de la pression et les pistes d'audit complètes sont passées du statut d'options souhaitables à celui d'exigences essentielles. Lors de l'évaluation de machines potentielles, il convient d'examiner attentivement la documentation du fournisseur, notamment les protocoles de qualification de la conception (DQ), de qualification de l'installation (IQ), de qualification opérationnelle (OQ) et de qualification des performances (PQ), car ces éléments ont un impact significatif sur les délais et les coûts de validation.
La force de compression appliquée lors de la formation des comprimés influe directement sur leurs caractéristiques de qualité essentielles, telles que la dureté, l'épaisseur, le temps de désintégration et le profil de dissolution. Les compresseurs à comprimés équipés de rouleaux de compression réglables avec précision permettent aux opérateurs d'ajuster ce paramètre avec exactitude afin de s'adapter aux différentes formulations, des comprimés fragiles et légèrement compactés aux compositions à haute densité nécessitant une pression importante.
Les presses modernes intègrent des systèmes de surveillance de la pression en temps réel qui mesurent en continu la force de pressage à chaque station, permettant ainsi la détection immédiate des écarts pouvant indiquer des irrégularités de remplissage, une usure des poinçons ou des modifications de la formulation. Les systèmes avancés peuvent ajuster automatiquement les paramètres afin de garantir la constance du remplissage ou rejeter les comprimés non conformes aux spécifications établies. Ce niveau de contrôle est particulièrement important pour les produits à marge thérapeutique étroite ou ceux soumis à des critères de bioéquivalence, pour lesquels de faibles variations peuvent impacter les performances cliniques.
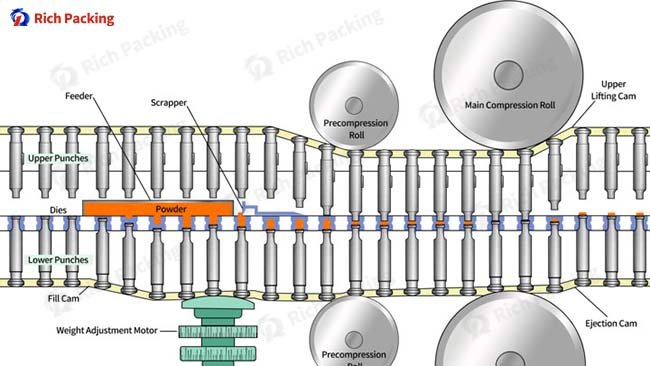
L'intégration des technologies d'automatisation représente une avancée majeure pour les presses à comprimés. Entièrement automatisées, ces presses améliorent non seulement le débit, mais aussi la constance du produit en réduisant l'intervention humaine dans les opérations courantes. Les systèmes automatisés de réglage en hauteur, les mécanismes d'indexation de la tourelle et les systèmes d'éjection contrôlée contribuent à un fonctionnement plus stable et à une moindre dépendance à l'opérateur.
Les systèmes de surveillance intelligents constituent une autre avancée majeure, permettant la mesure en temps réel du poids, de l'épaisseur et de la dureté des comprimés. Ces systèmes utilisent les principes du contrôle statistique des procédés pour détecter les tendances et alerter en amont des problèmes potentiels avant qu'ils n'entraînent le rejet de lots. Les systèmes les plus sophistiqués peuvent ajuster automatiquement les paramètres pour compenser les variations détectées, garantissant ainsi la qualité tout au long des cycles de production. Parmi les autres fonctionnalités importantes, citons les mécanismes de protection contre les surcharges qui préviennent les dommages causés par des particules étrangères ou un mauvais réglage, et les indicateurs de maintenance préventive qui suivent l'utilisation des composants et alertent les opérateurs lorsqu'une intervention est nécessaire.
Le processus de compression génère inévitablement de la poussière, ce qui peut compromettre la qualité du produit et la sécurité des opérateurs. Les systèmes de contrôle de la poussière efficaces utilisent des points d'aspiration stratégiquement placés aux points de transfert des matériaux et dans les zones de compression afin de capturer les particules en suspension dans l'air. Les zones de compression étanches et les circuits de matériaux fermés permettent de contenir davantage les contaminants potentiels, ce qui est particulièrement important pour les composés puissants ou les produits à faibles doses thérapeutiques.
Les considérations de sécurité ne se limitent pas au contrôle de la contamination et incluent la protection physique des opérateurs. machines de compression de comprimés L'installation intègre des systèmes de protection complets avec des points d'accès verrouillés qui interrompent le fonctionnement en cas d'ouverture. Des boutons d'arrêt d'urgence à plusieurs endroits, des barrières mécaniques autour des composants mobiles et des systèmes de limitation de pression préviennent les blessures par écrasement ou enchevêtrement. De plus, des protocoles de sécurité spécifiques sont essentiels lors de la manipulation de composés puissants, notamment des systèmes de décharge confinée et des capacités de nettoyage en place (NEP) qui minimisent l'exposition des opérateurs pendant les opérations de maintenance et de nettoyage.
Dans l'industrie pharmaceutique, les arrêts de production impactent directement les plannings et la rentabilité. Les machines conçues avec des composants modulaires et des systèmes de déconnexion rapide réduisent considérablement les temps de changement de lot. Le démontage sans outil pour le nettoyage et la maintenance améliore encore l'efficacité opérationnelle en accélérant la réalisation de ces opérations.
Le choix des matériaux de construction influe considérablement sur la facilité de nettoyage et la durabilité d'une structure. L'acier inoxydable de haute qualité, avec des finitions de surface appropriées, résiste à la corrosion et facilite la validation du nettoyage. Lors de l'évaluation de différents modèles, il convient de tenir compte de l'accessibilité des composants critiques tels que les rouleaux de compression, les systèmes d'alimentation et les ensembles de tourelle, car les zones difficiles d'accès peuvent prolonger les opérations de nettoyage et de maintenance. De plus, il est important d'évaluer la disponibilité des pièces de rechange, la réactivité du support technique et la réputation du fournisseur en matière de service après-vente, car ces facteurs ont un impact significatif sur la fiabilité opérationnelle à long terme.
Bien que le prix d'achat initial soit un facteur déterminant dans les décisions de sélection, une analyse financière complète doit prendre en compte le coût total de possession (CTP) tout au long du cycle de vie opérationnel de l'équipement. Cette approche globale inclut les coûts directs, tels que les contrats de maintenance préventive, les stocks de pièces de rechange et la consommation d'énergie, ainsi que les dépenses indirectes liées à l'efficacité opérationnelle, aux temps de changement de production et aux pertes de rendement.
Une machine nécessitant un investissement initial plus important peut offrir une valeur ajoutée supérieure à long terme grâce à des rendements de production plus élevés, des taux de rebut réduits, une consommation d'énergie moindre et des besoins de maintenance moins fréquents. À l'inverse, un équipement moins cher à l'achat peut engendrer des coûts d'exploitation plus élevés en raison d'un temps d'arrêt accru, d'un remplacement plus fréquent des composants ou d'une consommation d'énergie plus importante. Élaborez un modèle de coût total de possession (CTP) complet qui projette les coûts sur un horizon de 5 à 10 ans, en intégrant tous les facteurs pertinents afin de faire un choix financièrement judicieux et aligné sur les objectifs stratégiques de votre organisation.
1. Réalisez une analyse approfondie de vos besoins : avant de contacter les fournisseurs d’équipements, prenez le temps de documenter précisément vos besoins. Cela inclut les caractéristiques détaillées de la formulation, les volumes de production cibles, les contraintes de vos installations, les obligations réglementaires et vos projets d’expansion. Des exigences clairement définies facilitent les échanges avec les fournisseurs et garantissent que les solutions proposées correspondent à votre contexte opérationnel réel et non à des scénarios hypothétiques.
2. Privilégier la flexibilité opérationnelle et l'évolutivité : les portefeuilles de produits pharmaceutiques évoluent inévitablement au fil du temps, avec le lancement de nouveaux produits, les modifications de formulation et l'évolution de la demande du marché. Choisir un équipement doté d'une flexibilité intrinsèque permet de s'adapter plus efficacement à ces changements. Des caractéristiques telles que des tourelles facilement interchangeables, des systèmes d'alimentation modulaires et des plateformes de contrôle évolutives prolongent la durée de vie fonctionnelle de la machine et protègent votre investissement contre les aléas futurs.
3. Privilégier la gestion intégrée de la qualité : Les paradigmes modernes de la qualité mettent l’accent sur l’intégration de la qualité dans les processus, plutôt que de se fier uniquement aux tests sur les produits finis. Recherchez des équipements dotés de capacités de surveillance complètes qui fournissent des données de processus en temps réel et facilitent l’analyse des tendances. La capacité à démontrer une maîtrise constante des processus grâce à la collecte automatisée de données renforce votre position en matière de qualité lors des inspections réglementaires et accélère les décisions de libération des lots.
4. Réaliser une évaluation complète de l'intégration aux installations : au-delà des dimensions physiques de la machine, tenez compte de ses exigences d'intégration au sein de vos installations existantes. Évaluez les raccordements aux réseaux, les interfaces de manutention, les exigences d'accès du personnel et les dégagements nécessaires à la maintenance. Impliquer les parties prenantes des services techniques et d'ingénierie dès le début du processus de sélection permet d'identifier les éventuels problèmes d'intégration avant l'acquisition, évitant ainsi des modifications coûteuses ou des compromis opérationnels après l'installation.
5. Mettre en œuvre un protocole de tests de performance rigoureux : les spécifications théoriques offrent un aperçu limité par rapport aux performances observées en conditions réelles d’utilisation. Exigez la réalisation d’essais de production avec vos formulations spécifiques dans les locaux du fournisseur ou lors de démonstrations de l’équipement. Ces essais doivent simuler au mieux vos conditions d’utilisation prévues, notamment en incluant des tailles de lots représentatives, les procédures de changement de format et les protocoles de nettoyage. L’observation directe de la presse à comprimés manipulant vos matières premières fournit des données précieuses pour les décisions de sélection finales.
Q1 : Quels sont les facteurs spécifiques qui différencient les presses à comprimés à poinçon unique des machines rotatives à grande vitesse de fabrication de comprimés, au-delà de la capacité de production ?
A : Outre les différences évidentes de débit, ces catégories de machines présentent des caractéristiques de fonctionnement distinctes. Les presses à poinçon unique produisent généralement des comprimés moins durs en raison de leur compression intermittente, tandis que les presses rotatives offrent des étapes de précompression et de compression principale permettant d'obtenir des valeurs de dureté plus élevées. Les systèmes rotatifs garantissent généralement une meilleure uniformité de poids grâce à leurs mécanismes d'alimentation continue et à leurs temps de maintien contrôlés. De plus, les niveaux sonores, l'espace au sol requis et les compétences nécessaires de l'opérateur diffèrent considérablement entre ces technologies.
Q2 : Comment les presses à comprimés automatiques contribuent-elles spécifiquement à la conformité réglementaire ?
A: Les systèmes automatisés améliorent la conformité grâce à de multiples mécanismes : ils appliquent des contrôles de paramètres par limitation électronique des réglages, conservent des pistes d’audit complètes documentant tous les ajustements de processus, enregistrent automatiquement les données de contrôle qualité sans erreurs de transcription et réduisent la variabilité introduite par les opérations manuelles. Ces fonctionnalités soutiennent directement les principes d’intégrité des données énoncés dans les documents d’orientation réglementaires et facilitent les soumissions et les inspections réglementaires.
Q3 : Comment pondérer les critères d'évaluation lors de la comparaison de différentes options de machines à fabriquer des pilules ?
A : Bien que la pondération des facteurs varie selon les organisations, une approche équilibrée privilégie généralement la fiabilité technique (30 %), la conformité réglementaire (25 %), l'efficacité opérationnelle (20 %), le coût total de possession (15 %) et les capacités de support des fournisseurs (10 %). Cependant, ces pourcentages doivent être ajustés en fonction des circonstances spécifiques ; par exemple, les organisations manipulant des composés très puissants pourraient accorder une plus grande importance aux considérations de sécurité, tandis que celles opérant sur des marchés très concurrentiels pourraient privilégier davantage l'efficacité opérationnelle.
Choisir la machine de compression de comprimés adaptée à la production pharmaceutique est une décision stratégique cruciale, ayant un impact considérable sur la qualité des produits, l'efficacité opérationnelle et la conformité réglementaire. En abordant systématiquement les facteurs présentés dans ce guide – des spécifications techniques fondamentales à une analyse financière complète – vous établissez un cadre d'évaluation solide qui prend en compte à la fois les besoins immédiats et les objectifs à long terme.
Les choix les plus judicieux sont le fruit d'une approche collaborative impliquant les acteurs des fonctions de production, d'assurance qualité, d'ingénierie et de chaîne d'approvisionnement. Cette perspective multidisciplinaire garantit que l'équipement sélectionné répond aux exigences techniques tout en s'alignant sur la stratégie commerciale et les réalités opérationnelles. N'oubliez pas que la machine optimale représente non seulement un investissement, mais aussi un élément fondamental de votre capacité de production, qui influencera la performance et la compétitivité de votre organisation pour les années à venir. En appliquant rigoureusement ces principes, vous pourrez mener à bien le processus de sélection avec assurance et poser les bases d'une excellence manufacturière.